
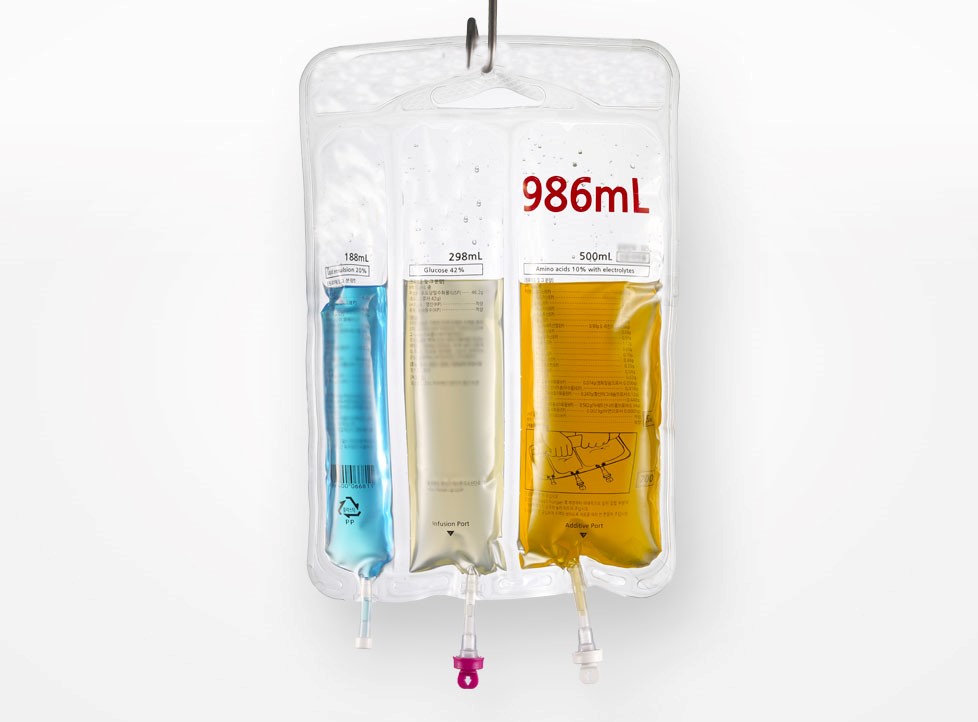
In today's rapidly developing global pharmaceutical industry, intravenous infusion (IV) therapy, as a key link in clinical medicine, has set unprecedented high standards for drug safety, stability, and production efficiency. The Multi Chamber IV Bag, with its unique compartment design, can achieve instant mixing of drugs and solvents, greatly improving medication accuracy and convenience. It has become the preferred packaging form for complex preparations such as parenteral nutrition, chemotherapy drugs, antibiotics, etc. However, the production of such products requires extremely strict requirements for equipment technology, clean environment, and compliance. Only engineering service providers with profound technical accumulation and global project experience can provide truly reliable solutions.
As an international leader in the field of medical engineering, IVEN Pharmatech Engineering, with over 30 years of experience in the pharmaceutical industry, is committed to providing global customers with one-stop turnkey engineering services from process design, equipment integration to compliance certification. Our Multi Chamber IV Bag Production Line not only integrates cutting-edge automation technology, but also has the core advantage of 100% compliance with international regulations such as EU GMP and US FDA cGMP, helping pharmaceutical companies efficiently create high value-added products and seize global market opportunities.
Multi Chamber IV Bag Intelligent Production Line: Redefining the Boundary between Efficiency and Safety
IVEN's multi chamber infusion bag production line is designed to meet the challenges of complex formulation production. Through four innovative technology clusters, it helps customers break through traditional production bottlenecks:
1. Multi chamber synchronous molding and precise filling technology
Traditional single chamber bags rely on external mixing steps, which pose a risk of cross contamination and are inefficient. IVEN adopts a multi-layer co extruded film material three-dimensional thermoforming process. Through high-precision molds and temperature gradient control, 2-4 independent chambers can be formed in a single stamping, with a partition strength of over 50N/15mm between chambers, ensuring zero leakage during transportation and storage. The filling process introduces a multi-channel filling pump driven by a magnetic levitation linear motor, with a minimum filling accuracy of ± 0.5%, supporting wide range adjustment from 1mL to 5000mL, perfectly adapting to the packaging needs of different viscosity fluids such as nutrient solutions and chemotherapy drugs.
2. Fully enclosed sterile connection system
To solve the problem of microbial control in pre mixed multi chamber bags, IVEN has developed a patented SafeLink ™ Aseptic activation device. The device adopts a laser pre cutting weakening layer design, combined with a mechanical pressure triggering mechanism. Medical staff only need to squeeze with one hand to achieve sterile communication between chambers, avoiding the risk of glass debris that may be generated by traditional folding valves. After third-party verification, the sealing performance of the activated connection meets the ASTM F2338-09 standard, and the probability of microbial invasion is less than 10 ⁻⁶.
3. Artificial intelligence visual inspection and traceability system
The production line integrates an AI X-ray dual-mode detection system, which synchronously detects film defects, filling liquid level deviations, and chamber sealing integrity through high-resolution CCD cameras and micro focus X-ray imaging. Deep learning algorithms can automatically identify pinhole defects at the 0.1mm level, with a false detection rate of less than 0.01%. At the same time, each infusion bag is implanted with an RFID chip to achieve full traceability from raw material batches, production parameters to circulation temperature, meeting the serialization requirements of the FDA DSCSA (Drug Supply Chain Safety Act).
4. Energy saving continuous sterilization solution
The traditional intermittent sterilization cabinet has pain points of high energy consumption and long cycle. IVEN and its German partners have jointly developed the Rotary Steam in Place (SIP) system, which adopts a rotating spray tower design to create turbulence in the superheated steam chamber. It can complete sterilization within 15 minutes at 121 ℃, saving 35% energy compared to traditional methods. The system is equipped with a self-developed B&R PLC controller, which can record and store the thermal distribution data of each batch (F ₀ value ≥ 15), and automatically generate electronic batch records that comply with 21 CFR Part 11.
IVEN's commitment: a global service network centered on customer success
We are well aware that first-class equipment needs to be matched with first-class service. IVEN has established technical centers in 12 countries worldwide, providing 7 × 24-hour remote diagnosis and 48 hour on-site response support. Our team can provide customized after-sales services based on the differences in regulations in different regions.
In the era of precision medicine and personalized medication, multi chamber intravenous infusion bags are reshaping the boundaries of parenteral treatment. IVEN Pharmatech Engineering builds a bridge to the future for global pharmaceutical companies with its outstanding engineering expertise and ultimate pursuit of compliance. Whether it's new projects or capacity upgrades, our intelligent production line will become your most trusted partner.
Contact the IVEN expert team immediately for customized solutions and global success stories!
Post time: May-27-2025


